Background: Clonal cytopenia of undetermined significance (CCUS) is characterized by the presence of a clone with somatic mutations in genes associated with myeloid neoplasms (MN) or abnormal cytogenetics, along with unexplained and persistent cytopenias in the absence of significant marrow dysplasia. CCUS is considered a premalignant state with a risk of progression to MN. The clinical outcomes of patients (pts) with high risk CCUS (Weeks et al, NEJM Evidence 2023) may be similar that of some patients with myelodysplastic syndromes (MDS). This study aims to predict the outcomes of CCUS patients using the molecular international prognostic scoring system (IPSS-M) developed for MDS.
Methods: A retrospective analysis was conducted in patients diagnosed with CCUS between 2015 and 2023, we included patients harboring pathogenic mutations (MUT) identified through next-generation sequencing (NGS) testing at two institutions: Mayo Clinic and the University of Texas Southwestern Medical Center, Dallas, TX, USA were included. Progression was considered present if patients with CCUS progressed into myeloid neoplasm at follow up. Statistical analysis was performed using JMP® 17.1.0 Software. IPSS-M risk scores were calculated via the IPSS-M Risk Calculator (mds-risk-model.com).
Results:
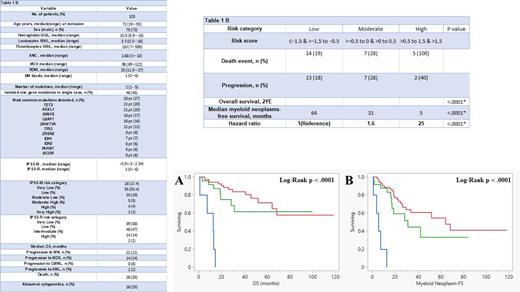
A total of 103 patients were included in the study. The median age was 72 years, the majority were male patients (73%), and the median bone marrow blast % at the time of NGS was 1%. Forty-five % had an isolated mutation in a single gene, and 55% had co-mutations in ≥1 gene. The median number of mutations was 2 (range 1 - 5), occurring across 43 different genes. The most common mutations were in TET2 (N=28; 27%), ASXL1 (N=21; 20%), SRSF2 (N=21; 20%), U2AF1 (N=18; 17%), DNMT3A (N=16; 16%), and TP53 (N=12; 12%). Additionally, 25% of patients had an abnormal karyotype. Hematologic and genetic characteristics are summarized in (Table 1A). The median IPSS-M score was -0.9 (-3 to +2.34) and the median IPSS- Revised (R) score was 2 (0 - 6). The majority of patients had low (L) IPSS-M risk category (53%), followed by moderate low (ML) (19%) and very low (VL) (17%). Per IPSS-R, the majority had low-risk category (47%), followed by VL (38%) and intermediate (14%).
Overall, 26 (25%) pt died after a median follow-up of 34 months and 21% progressed to MN (MDS 14 & CMML 8), and 1 patient with MDS and 1 with CMML progressed to secondary AML (2%). The median overall survival (mOS) was not reached, with a 2-year estimated (YE) OS and leukemia-free survival (LFS) of 80%.
Predictive Power: When utilizing the IPSS-M risk category ( L, M, H) * by combining the risk categories into L *(L+VL), M * (ML+MH), and H *(H+VH) to overcome the low sample size limitation, it significantly predicted OS (p < .0001) ( Figure 1A) and median MN-free survival (FS) (p < .0001) ( Figure 1B). The 2YE-OS rates by IPSS-M risk category were as follows: VL - 93%, L - 87%, ML - 75%, high (H) - 25%, very high (VH) - 0% and the 2YE-OS rates by IPSS-M ( L, M, H) are summarized in Table 1B. IPSS-M hazard ratio (HR) for OS was 1.6 for ( M*) compared to 25 for ( H *) risk category (p < .0001). The ( L *) category, being the largest, was used as the reference level.
Conclusion: This study provides the first real-world experience of using the IPSS-M and a modified version of the IPSS-M ( L, M, H) * risk category model to predict survival and progression into MN in CCUS (MUT) pt. The IPSS-M risk category was found to be a significant predictor of OS and MN-free survival. We propose that larger cohorts are needed to validate our findings.
Disclosures
He:Kura Oncology, Inc: Consultancy. Patnaik:StemLine: Research Funding; CTI Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees; Kura Oncology: Research Funding. Alkhateeb:Mayo Clinic: Current Employment. Madanat:Blueprint Medicines: Consultancy, Honoraria, Other: travel reimbursement; GERON: Consultancy; Sierra Oncology: Honoraria; Morphosys: Honoraria, Other: travel reimbursement; Stemline therapeutics: Honoraria; Taiho oncology: Honoraria; Novartis: Honoraria; OncLive: Honoraria; MD Education: Honoraria; Rigel Pharmaceuticals: Honoraria.